Flexibilité structurale des biomolécules en milieux complexes
Structural flexibility of biomolecules in complex environmentsResearch
Paramagnetic labels for in-cell EPR studies & strategies for cellular sample preparation
Our research group is focused on the development of next-generation nitroxide labels optimized for the intracellular environments. These part of the research activities aims to obtain optimal nitroxide labels able to probe protein dynamics, conformations and interactions inside cells. At the same time, our team are currently elaborating different strategies aiming to deliver spin labelled proteins in cell for EPR measurements, using sevral approaches as electroporation and heat-shock.
Another part of our research is centered on the development of new intracellular SDSL strategies based on AMBER suppression technologies for the incorporation of noncanonical amino acids (nCAAs) onto proteins. In order to carry out bio-orthogonal labelling approaches, we mainly adopt spin labels (nitroxides and Gd(III)-tags) which cab be selectively grafted on proteins via click-chemistry reactions. In this context, we are also working to integrate genome-editing technologies (i.e. CRISPR-cas) with these intracellular SDSL methodologies to perform in-cell EPR experiments at real physiological conditions.
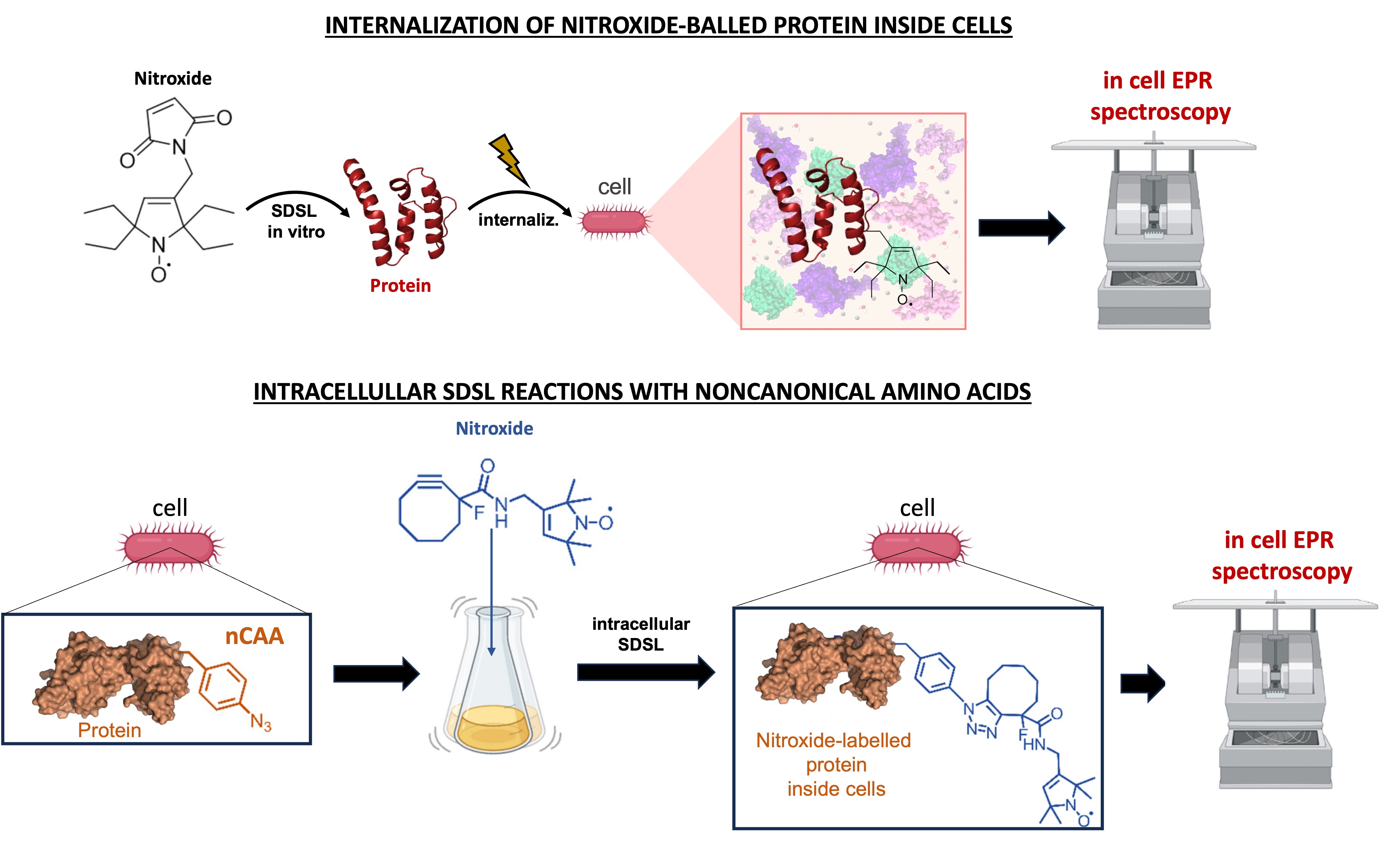
In-cell EPR spectroscopy to probe protein dynamics and interactions
Our team uses high-resolution EPR measurements (in vitro & in cell) to analyze conformational ensembles and interaction networks of proteins where the intrinsic structural flexibility plays a pivotal role on their functionalities. We mainly apply cw-EPR spectroscopy to probe protein dynamics at local level of different systems in simple buffer and in real cellular environments. In parallel, our laboratory exploits pulse EPR measurements (i.e. DEER & RIDME experiments) with spin labelled proteins to monitor conformational motions and oligomeric complexes.
We develop and apply SDSL reactions with nitroxide which can be selectively incorporated onto different residues (cysteine, tyrosine, nCAA, etc.) for EPR experiments. Moreover, other classes of spin labels (i.e Gd (III)-based tags) and orthogonal-SDSL reactions are also currently involved in our research project activities to study protein structures and interactions
 .
.
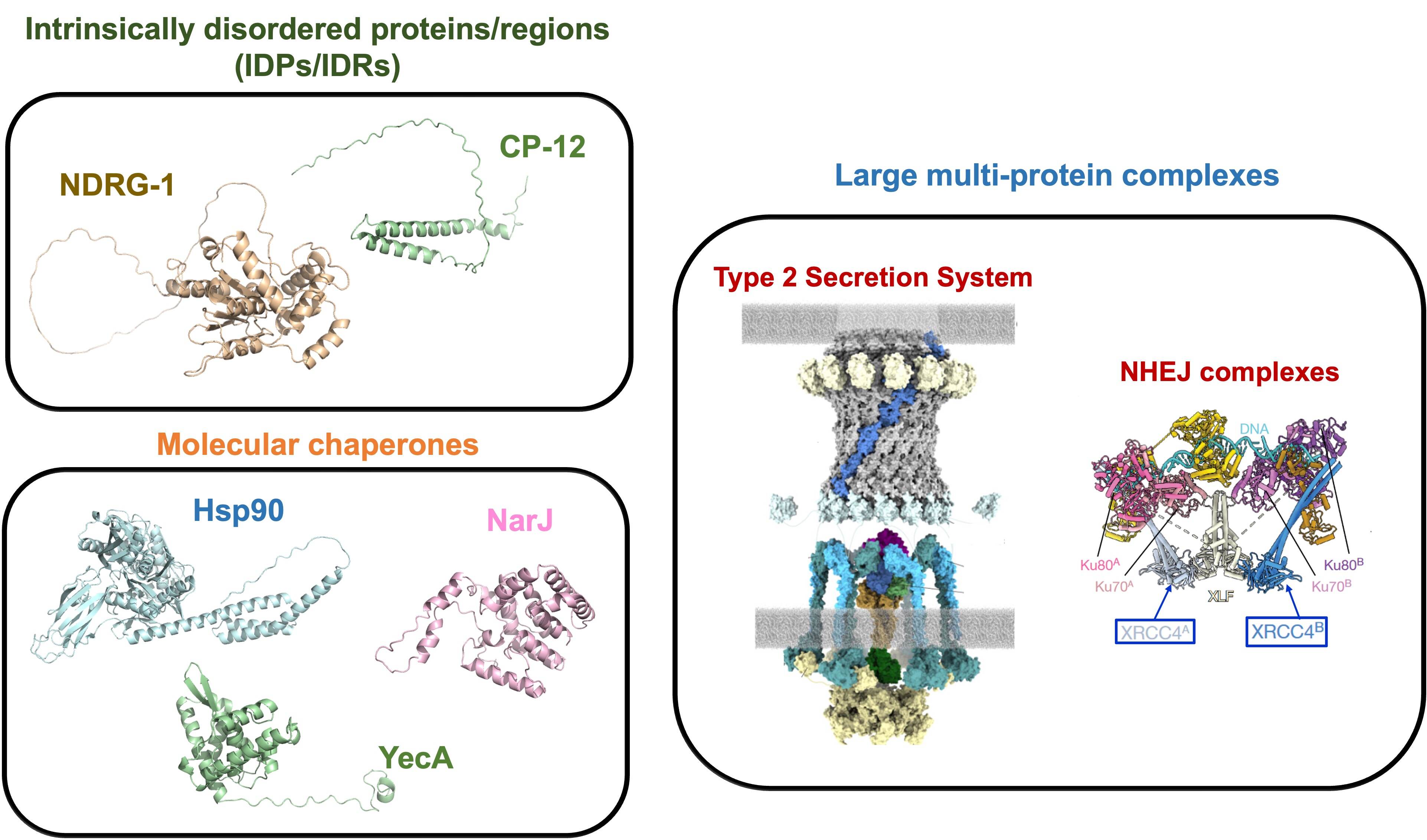
Biological systems under investigation
Our research team is particularly interested on the evaluation on the structure-function relationship of highly flexible proteins. A part of our activities focused on the evaluation of a group of proteins, such as Intrinsically Disordered Proteins (IDPs) and molecular chaperones, where the conformational plasticity play a pivotal role in their functionalities. We are also focusin our research on the investigation of the biogenesis and the network of interactions (protein-protein, protein-DNA, etc.) acting in large multi-protein complexes.
Finally, we also collaborate with national & international laboratories to integrate SDSL-EPR experiments with other biophysical techniques (NMR, X-ray crystallography, Cryo-EM, SAXS, Molecular Dynamic simulations, etc.) to solve open questions in the context of structural biology of proteins.
Biophysical techniques
- X-band cw-EPR spectroscopy
- Q-band & W-band pulse EPR spectroscopy (i.e. DEER & RIDME experiments)
- solution-state NMR spectroscopy
- Flow Cytometry
- Fluorescence Microscopy
- Protein engineering
- Protein chromatography
Scientific collaborations
- Olivier OUARI (ICR, Aix-Marseille Univ., France)
- Raffaele IEVA (LMGM/CBI, CNRS-Tolouse, France)
- Barbara ZAMBELLI (Univ. di Bologna, Italy)
- Luca LIGNITTO (CRCM, Inserm Marseille, France)
- Thierry DOAN (LISM, CNRS Marseille, France)
- Olivier GENEST (BIP, CNRS Marseille, France)
- Romé VOULHOUX (LCB, CNRS Marseille, France)
- Flavio DI PISA (IBF, CNR-Milano, Italy)
- Simone CIOFI BAFFONI (CERM, Univ. di Firenze, Italy)
- Matteo DE ROSA (IBF, CNR-Milano, Italy)
- Pascale BARBIER (INP, Aix-Marseille Univ., France)
- Irene DIAZ MORENO (Univ. de Sevilla, Spain)
- Takahisa YAMATO (Nagoya Univ., Japan)
- Fabien FERRAGE (CNRS & ENS Paris, France)
- Mauro MODESTI (CRCM, Inserm Marseille, France)
- Caroline SMET-NOCCA (CNRS, Inserm & Univ. Lille, France)
- Veronique RECEVEUR-BRECHOT (BIP, CNRS Marseille, France)
- Deborah Byrne (IMM, CNRS-Marseille, France)
