Bioélectrochimie, biointerfaces et biotechnologies
Bioelectrochemistry, biointerfaces and biotechnologiesMethods
Microscopy
Cyclic Voltammetry
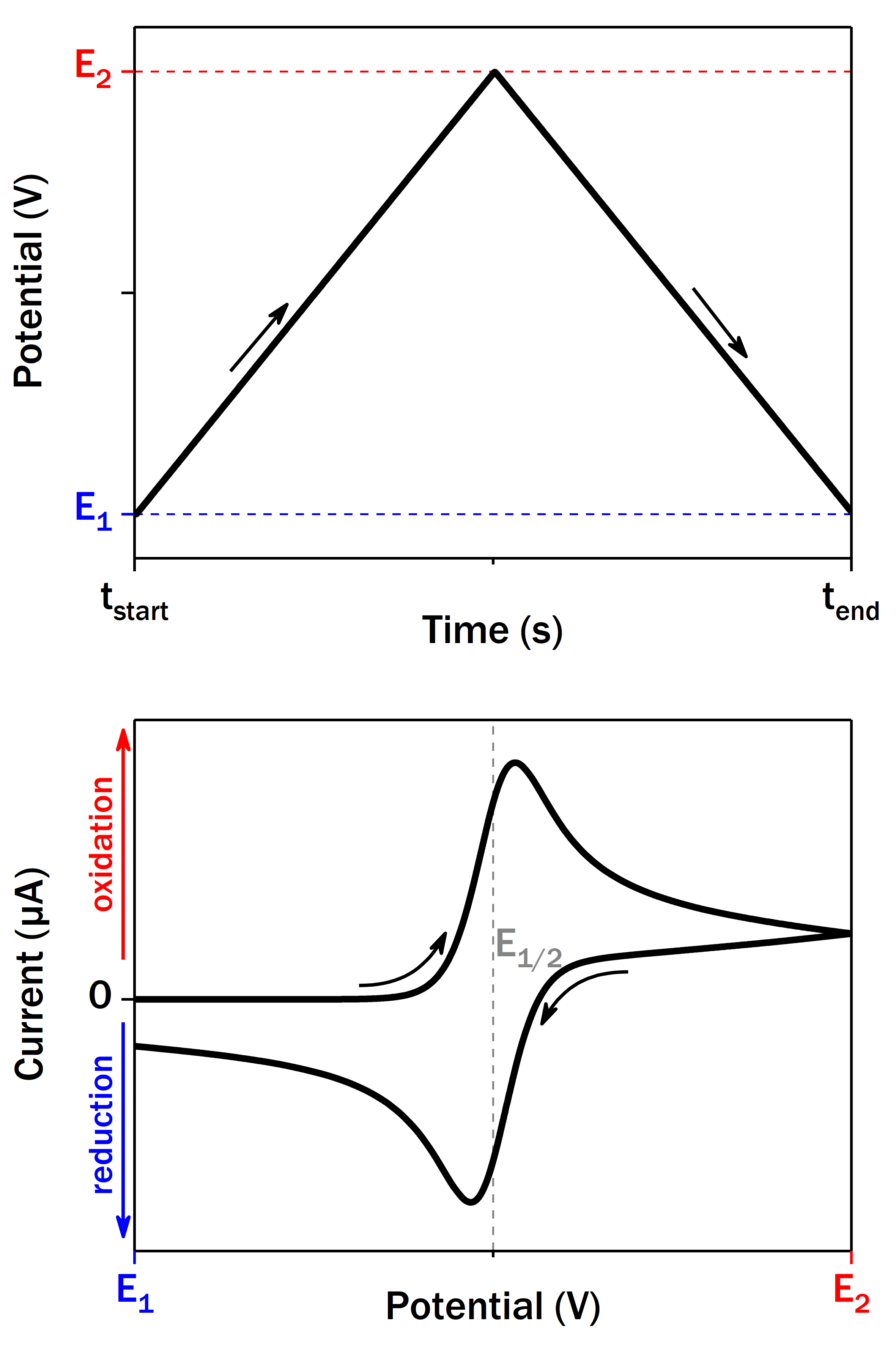
Cyclic voltammetry (CV) is a common electrochemical method used to study redox systems diffusing in the solution or adsorbed on the electrode surface. A typical setup consists of three electrodes: working electrode (WE), reference electrode (RE) and counter electrode (CE). The potential of WE (versus RE) is changed linearly with time between two border values E1 and E2 at a certain scan rate. The current passing between WE and CE is recorded, which may be positive (oxidation) or negative (reduction). The resulting current vs potential graph (voltammogram) depends on the nature of redox system. For the simplest fast one-electron redox system in the solution it represents two symmetric waves with peak-to-peak separation close to 57 mV at 25 °C. The midpoint potential (E1/2) between two peak is characteristic of the redox couple.
Upon careful experiment design CV can give a multitude of information about the thermodynamics (potential) and the kinetics (current) of the redox system. CV of adsorbed redox proteins has some peculiarities and it is often called protein film voltammetry. In the absence of enzyme substrate and when the electron exchange rate between the biomolecule and electrode is fast enough, an enzyme monolayer should give a pair of symmetric peaks whose charge is proportional to the enzyme coverage. These peaks are virtually undetectable for most of the large enzymes though, due to the consequent low coverage. The signals can be significantly amplified in the presence of enzyme substrate(s) giving rise to so-called catalytic current.
Pulsed Methods
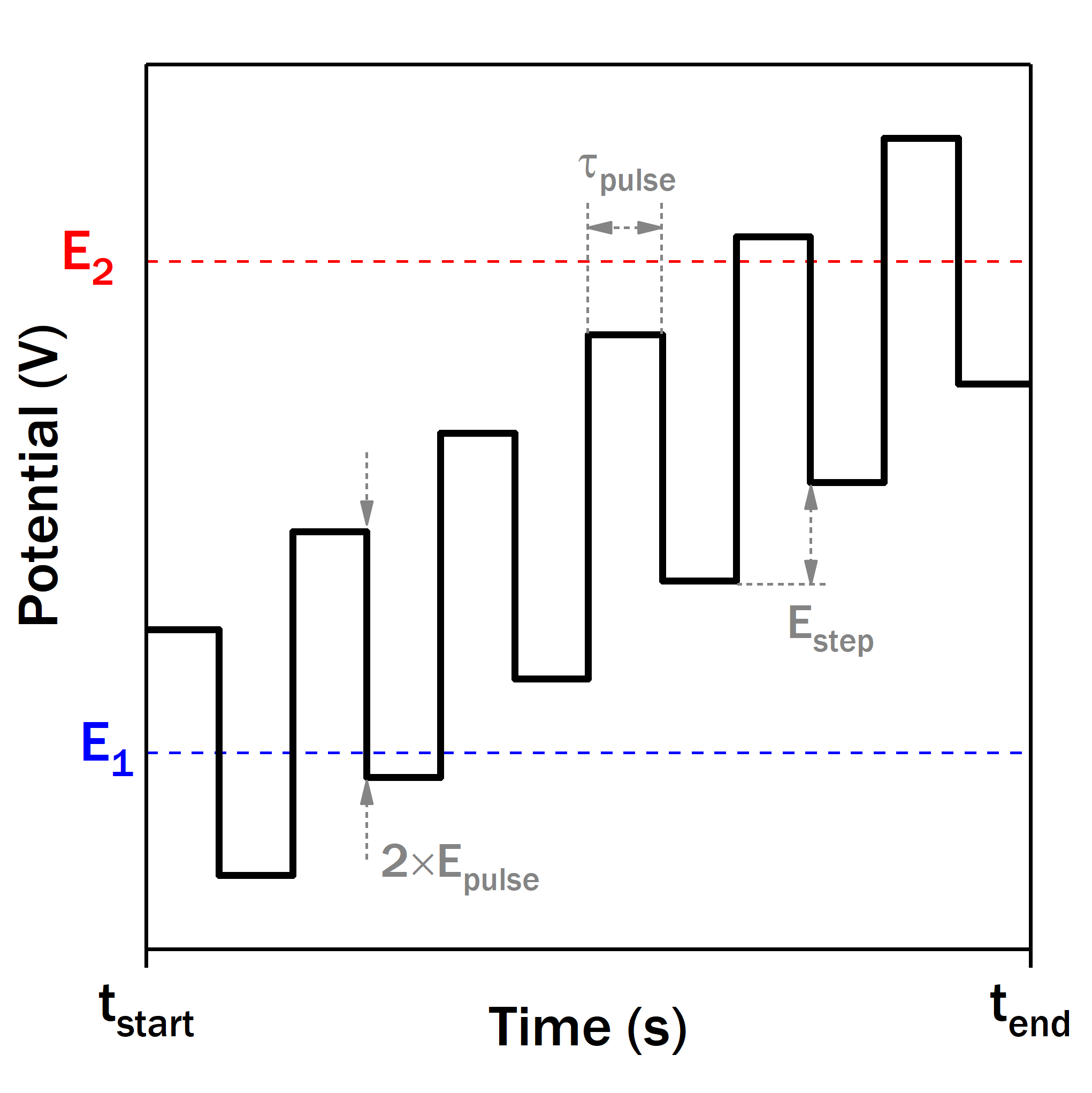 Besides a faradaic current from the redox system of interest, CV always has a contribution of non-faradaic current caused by the formation of the electric double-layer on the electrode boundery, so-called capacitive current. Depending on the electrode material and surface area (applicable to nanostructured electrodes in particular), capacitive current may reach high values and completely mask the faradaic response on CV.
Besides a faradaic current from the redox system of interest, CV always has a contribution of non-faradaic current caused by the formation of the electric double-layer on the electrode boundery, so-called capacitive current. Depending on the electrode material and surface area (applicable to nanostructured electrodes in particular), capacitive current may reach high values and completely mask the faradaic response on CV.
Different formes of potential rampe can be used in such case to increase the contribution of the faradaic current compared with the capacitive one. All such methods make use of different kinds of potential pulses for this purpose, e.g. differential pulse voltammetry (DPV) and squarewave voltammetry (SWV). Thanks to the particular current sampling, the resulting voltammogram has minimal capacitive current contribution while the faradaic current waveform has broad diagnostic use.
Electrochemical Surface Plasmon Resonance (e-SPR)
Surface plasmon resonance (SPR) is the resonant oscillation of conduction electrons at the interface between negative and positive permittivity material stimulated by incident light. Mostly used with gold, a common SPR setup consists of the thin gold film deposited onto a glass prism. A laser illuminates the backside of the film through the prism at the conditions of total internal reflection. This creates an evanescent wave that excites surface plasmons propagating in the direction parallel to the boundary between the gold film and the solution. Since this wave is surface-confined, it is sensitive to all changes on the boundary, for example, adsorption or desorption of the molecules.
 The gold film can act as working electrode in the same time. Hereby, all the surface changes occurring on the timescale of electrochemical experiment will be recorded as SPR-signal. This allows to follow in situ multiple processes of interest, such as SAM formation and protein adsorption, desorption or reorganization. Coupling of electrochemistry and SPR also enables the observation of the bioelectrode in operando, during the catalytic turnover. The correlation between the activity and the redox enzyme amount on the surface, which is not always straightforward, allows us to further decipher the molecular bases of the bioelectrocatalysis.
The gold film can act as working electrode in the same time. Hereby, all the surface changes occurring on the timescale of electrochemical experiment will be recorded as SPR-signal. This allows to follow in situ multiple processes of interest, such as SAM formation and protein adsorption, desorption or reorganization. Coupling of electrochemistry and SPR also enables the observation of the bioelectrode in operando, during the catalytic turnover. The correlation between the activity and the redox enzyme amount on the surface, which is not always straightforward, allows us to further decipher the molecular bases of the bioelectrocatalysis.
Electrochemical Quartz Crystal Microbalance (e-QCM)
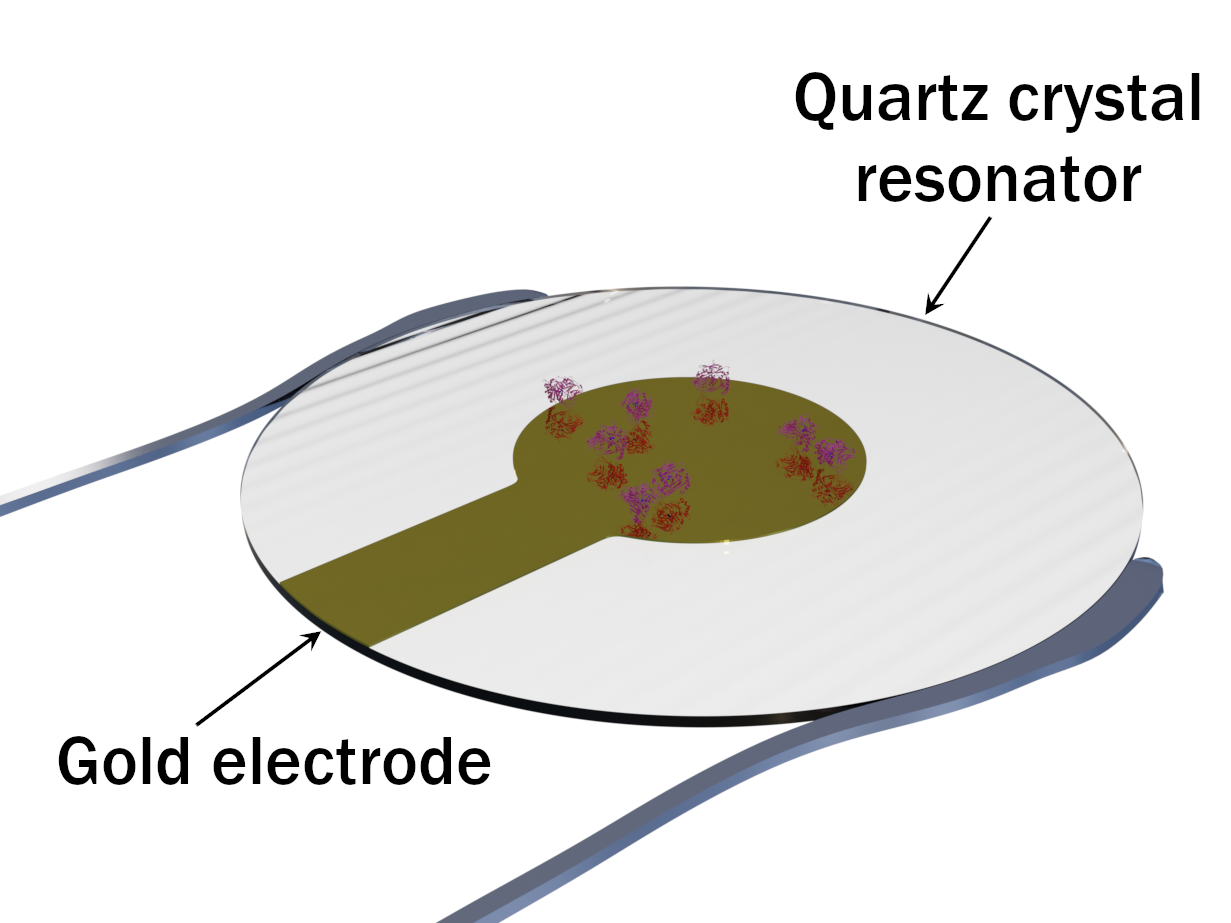 Quartz Crystal Microbalance (QCM) can be considered as an alternative to SPR for detection of surface sorption processes. Its principle is based on the change of resonance frequency of quartz crystal resonator upon its mass change due to the protein adsorption. Masses as small as 1 µg/cm2 can be detected.
Quartz Crystal Microbalance (QCM) can be considered as an alternative to SPR for detection of surface sorption processes. Its principle is based on the change of resonance frequency of quartz crystal resonator upon its mass change due to the protein adsorption. Masses as small as 1 µg/cm2 can be detected.
Using a thin gold electrode attached to the quartz crystal, QCM can be coupled to electrochemistry in order to study the variation of adsorbed redox enzyme mass during an electrochemical experiment.
Microscopy
Fluorescence microscopy under electrochemical potential control
🇬🇧 Fluorescence confocal laser scanning microscopy is a powerful optical technique that allows for the non-invasive optical sectioning of a volume. In the confocal scanning mode, light is collected at only one plane of focus through a small adjustable pinhole. The pinhole eliminates the out-of-focus light and thus increases the image resolution, laterally and axially. By scanning across multiple planes of focus, optical slices of the sample can be obtained and 3D structure of the sample (the enzymatic electrode in our case) can be visualised using 3D image reconstruction software.
 In situ-coupling of e-fluorescence microscopy enables us to collect global electrochemical information about enzymatic processes as well as the corresponding local microscopic data at the electrode surface/volume simultaneously. This allows for the study of enzyme repartition and the catalytic heterogeneities at the electrode. Enzyme reactivity at the surface of the electrode can be characterized via e-fluorescence microscopy, using a fluorophore whose fluorescence is modulated by the enzymatic reaction.
In situ-coupling of e-fluorescence microscopy enables us to collect global electrochemical information about enzymatic processes as well as the corresponding local microscopic data at the electrode surface/volume simultaneously. This allows for the study of enzyme repartition and the catalytic heterogeneities at the electrode. Enzyme reactivity at the surface of the electrode can be characterized via e-fluorescence microscopy, using a fluorophore whose fluorescence is modulated by the enzymatic reaction.
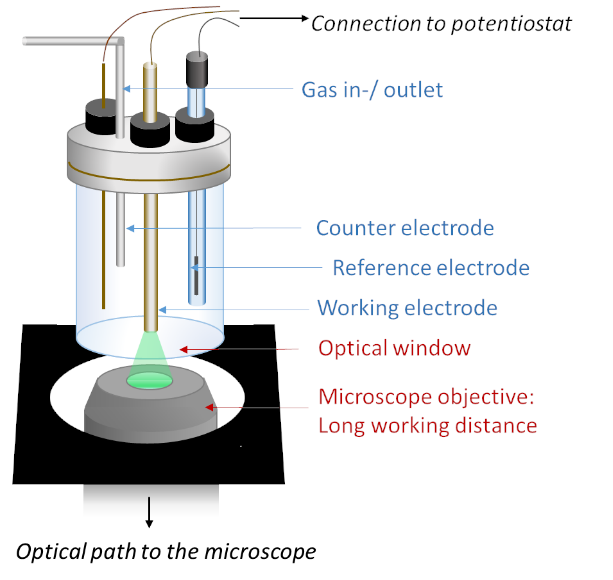
The experimental setup of e-fluorescence microscopy consists of a three-electrode spectro-electrochemicall cell placed directly above the objective of the microscope, under electrochemical control (by a potentiostat and a computer). As light passes through the objective and then the thin optical window of the spectro-electrochemical cell, it is then focused on the enzymatic electrode. The experimental setup can be adapted for different macro- and micro-electrodes conveniently.
🇫🇷 La microscopie photonique confocale est une méthode optique puissante, qui permet de découper de manière non invasive un volume en « tranches optiques ». En mode confocal, la fluorescence est collectée exclusivement dans un fin volume centré autour du plan focal grâce à un « pinhole » placé dans le chemin optique. Ce pinhole empêche les rayons lumineux qui ne sont pas issus du plan focal d’accéder au détecteur, ce qui diminue le flou et augmente à la fois la résolution latérale et la résolution axiale. En enregistrant la fluorescence à divers plans focaux parallèles les uns aux autres, on peut ainsi définir plusieurs « tranches optiques » d’un échantillon (dans notre cas, l’électrode enzymatique ou la solution avoisinante), et reconstruire sa structure 3D grâce à des logiciels de traitement d’images.
Le couplage in situ de la microscopie de fluorescence confocale et de l’électrochimie nous permet de caractériser les processus enzymatiques en enregistrant simultanément des informations électrochimiques à l’échelle globale de l’électrode, et les données microscopiques locales correspondantes. Ceci permet d’étudier la répartition de l’enzyme à la surface (ou dans le volume) de l’électrode ; ou la distribution des hétérogénéités de la catalyse, et donc de caractériser l’activité enzymatique locale.
Les expériences sont réalisées grâce à une cellule « opto-électrochimique » à trois électrodes, c’est-à-dire une cellule à trois électrodes possédant un fond transparent optiquement (épaisseur : 170 µm). Celle-ci est placée au-dessus de l’objectif du microscope confocal inversé. Une station électrochimique mobile (potentiostat et ordinateur) permet de réaliser les mesures sous contrôle électrochimique. Le montage expérimental peut-être adapté à souhait pour les différentes macro- ou micro-électrodes.
Self-assembled monolayers (SAM)
SAM are ordered molecular assemblies formed by chemosorption on the electrode surface. The self-organization of aliphatic thiol-containing molecules on gold surface is the well-known example. The SAM-modified Au-electrodes have been largely studied in electrochemistry.

In our group we use thiol-molecules with various end-groups, such as carboxy- or amine moieties. When an enzyme adsorbes on SAM, it may adopt different conformation and orientation depending on the interactions of polypeptide backbone with the terminal SAM groups. These factors, together with the length of SAM aliphatic backbone, influence the rate of interfacial electron transfer between enzyme and electrode.
Carbon nanotubes (CNT)
 CNT are cylindrical tubes made of carbon with diameters in the nanometer range. They can also be imagined as graphene sheet(s) rolled up along one of the lattice vectors. CNT may consist of one graphene sheet, single-wall CNT (SWCNT), or several nested sheets, multi-wall CNT (MWCNT). CNT have several unique properties, such as thermal and electrical conductivity, high surface area, tensile strength, which make them promising in many scientific domains. For electrochemistry, MWCNT are more useful since they always possess metallic conductivity and their outer wall can be chemically modified without losing the conductivity.
CNT are cylindrical tubes made of carbon with diameters in the nanometer range. They can also be imagined as graphene sheet(s) rolled up along one of the lattice vectors. CNT may consist of one graphene sheet, single-wall CNT (SWCNT), or several nested sheets, multi-wall CNT (MWCNT). CNT have several unique properties, such as thermal and electrical conductivity, high surface area, tensile strength, which make them promising in many scientific domains. For electrochemistry, MWCNT are more useful since they always possess metallic conductivity and their outer wall can be chemically modified without losing the conductivity.
 We use MWCNT for electrode modification before adsorbing the enzymes. This produces a suitable porous and conductive matrice for biomolecule immobilisation that can accommodate much higher amount of enzymes than a planar electrode, thus enhancing the biocatalytic current density. Furthermore, covalent or non-covalent (p-p stacking) modification of CNT walls can be used to tune the electrostatic interactions with biomolecules or to chemically link enzymes.
We use MWCNT for electrode modification before adsorbing the enzymes. This produces a suitable porous and conductive matrice for biomolecule immobilisation that can accommodate much higher amount of enzymes than a planar electrode, thus enhancing the biocatalytic current density. Furthermore, covalent or non-covalent (p-p stacking) modification of CNT walls can be used to tune the electrostatic interactions with biomolecules or to chemically link enzymes.
Performance of the enzymatic bioelectrodes depends on multiple factors, such as local substrate concentration and pH, solution conductivity or enzyme inhibition. These factors are often interdependent and all together contribute to the final shape of voltammograms and chronoamperograms. When the enzyme is immobilized on a planar electrode, the experiments can be carried out in the conditions allowing to neglect influence of certain factors. For example, electrode rotation allows omitting the diffusional component, which significantly simplifies the situation and often permits to deduce an analytical solution to the equations.

However, such factors can’t be neglected when the porous electrodes of complex geometry are used and/or when the high enzymatic activity results in rapid concentrations change near the bioelectrode. Analytical solution being impossible in such cases, a numerical modelling and simulation of the whole electrochemical system can be used to take into account all the factors. To simulate such complex systems, we often use the finite element method (FEM) implemented in the COMSOL Multiphysics software package. In the FEM, the geometry is subdivided into smaller simpler parts that are called finite elements. The global problem is then approximated into a system of algebraic equations for each finite element, which is solved by the software. This method allows to predict current-voltage characteristics for complex bioelectrochemical systems taking into account enzyme kinetics, local pH and diffusion of all species. This information can be further used for the performance improvement of bioelectrodes.
