Evolution de la bioénergétique
Evolution of bioenergeticsWhat is "Free Energy"?
and what does it have to do with Life's Emergence and the Evolution of
Complexity?
and what does it have to do with Life's Emergence and the Evolution of Complexity?
Thermodynamic considerations allow deducing the general framework of environmental conditions permitting life to emerge and to persist. The fundamental conditio sine qua non is the existence of some kind of thermodynamic disequilibrium able to fuel local entropy decreases via disequilibria-converting mechanisms. The research field studying such mechanisms employed by life is called Bioenergetics. The comparative survey of bioenergetic principles operating in cellular life permits narrowing down the general thermodynamic requirements towards the specific conditions which likely drove the emergence of life on our planet.
(I) The nature of the driving disequilibria: The specific thermodynamic disequilibrium sustaining all non-photosynthetic life on Earth is the electrochemical tension, that is, a redox disequilibrium between reducing and oxidising environmental substrates (see Schoepp-Cothenet et al., 2013). Bioenergetic electron transfer chains convert this electrical potential into a proton (or sodium) motive force (composed of electrial field and osmotic components) which eventually is converted to a chemical disequilibrium, that is, ATP/ADP (or PPi/Pi) ratios displaced far from equilibrium.


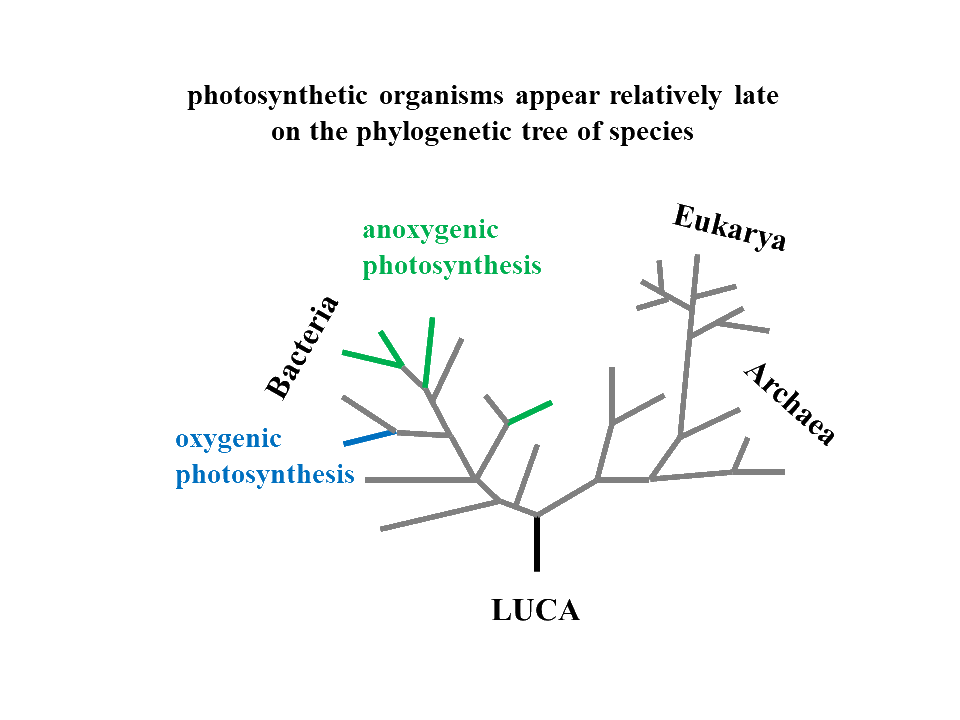
In anoxygenic photosynthesis, the terminal environmental oxidant in the above described scheme is replaced by a light-induced positive charge while in oxygenic photosynthesis, both environmental oxidant and reductant are produced by tapping into the energy of visible-light photons (see figure below). The remaining components of the full photosynthetic electron transfer chains fully correspond to those of non-photosynthetic organisms. Phylogeny of the respective enzymes as well as the occurrence of phototrophs on the phylogenetic tree of life strongly indicate that chemotrophic life preceeded phototrophic one and that photosynthetic reaction centres were additions to pre-existing electron transfer chains collapsing environmental redox disequilibria.

In fermentation the electron sink is generated by continuously excreting partially or fully reduced metabolites (such as ethanol, aldehyde, formate, methane, acetate etc). In many of these cases, the free energy contained in the respective redox reactions is insufficient to participate in membrane-potential generation and increasing ATP-levels therefore has to proceed via substrate-level-phosphorylation.
Conclusion: The layout of extant life indicates that the fundamental source of free energy (i.e. the environmental disequilibrium) driving the entropy-decrease characterising living cells is electrochemical. We have no reason to assume that this may have been different when life emerged. However, while the proton- (sodium-) motive potential in extant cells is converted from the electrochemical potential, there is reason to believe that during life’s emergence(in specific locales, see AHV-hypothesis), a proton disequilibrium may have been constitutive and thus have served as a source of free energy in its own right next to the environmental electrochemical potential.
(II) On the importance of 2-electron electrochemistry:
A significant fraction of biologically relevant redox centres are 2-electron compounds. In aqueous solution (due to the possibility of proton-coupled electron tranfer) and under appropriate conditions (e.g. pH value), such 2-electron compounds can exhibit cooperative redox behaviour (see 2-electron webpage), in which both electrons come or go together rather than one-by-one in a consecutive manner. The existence of cooperative redox behaviour entails two corrolaries arguably crucial for life:
1. Metastable redox gradients. Cooperative 2-electron compounds react very sluggishly with 1-electron centres even if the overall electron transfer reactions are strongly exergonic. This property allows the build-up of metastable electrochemical gradients in aqueous solutions, a prerequisite for life being able to harvest the free energy inherent in these electrochemical disequilibria. Present day examples for such metastable environmental disequilibria are provided by the coexistence of molecular hydrogen or methane in the presence of oxygen. On the early Earth, ferrous iron and nitrate would have redox-equilibrated very slowly allowing for the build-up of significant concentrations of the environmental oxidant nitrate (see also Wong et al. 2017). In living cells the extremely strong redox cooperativity of the NAD(P)H/NAD(P)+ couple prevents NAD(P)H from becoming non-enzymatically oxidised in the presence of multiple 1-electron acceptors such as for example non-heme iron centres or iron-sulphur clusters.
2. Up-conversion of reducing power via electron bifurcation. As detailed on our 2-electron webpage (see also Baymann et al., 2018), 2-electron compounds featuring strong positive redox cooperativity are capable of generating 1-electron reductants more reducing than the initial 2-electron redox centre. This reaction is driven by the overall exergonic ΔG of the overall reaction (see Figure below). Prominent biological examples comprise the Qo-site reaction of Rieske/cytb complexes (Bergdoll et al. 2016) or the flavin-based electron bifurcations as reviewed in Baymann et al. 2018. We are presently exploring the possibility whether the 2-electron transition metals Molybdenum and Tungsten are able to perform electron bifurcation (Duval et al. 2016) and thereby could have performed, during life’s emergence, the redox up-converting roles played by quinones and flavins after the organic take-over.
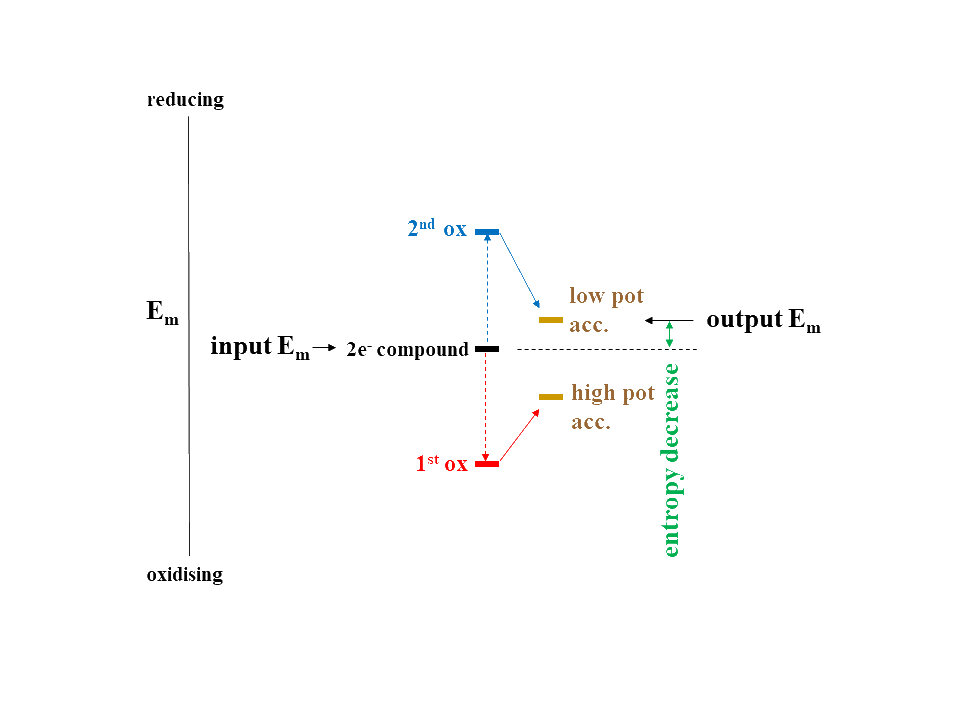
The generation of a lower potential reductant represents a local increase of redox disequilibrium (and therefore a local decrease in entropy) and the mechanism of electron bifurcation may therefore be part of the ancestral repertoire of disquilibrium converters driving the emergence of life.
We emphasize that both metastable redox gradients and the process of electron bifurcation do only exist in protic solvents (or if the protein-environment surrounding the 2-electron centre contains deprotonatable groups with appropriate pK-values). This fact may rationalise why water appears to be an indispensable ingrediant for the emergence of life.
(III) Conversion of environmental disequilibria into the entropy-decrease characterizing life; the bioenergetics-mineral connection:
Again, general thermodynamic considerations tell us that, while environmental disequilibria (or more specifically, redox gradients, see above) are the ultimate drivers of life’s emergence, we also need to elucidate the types of mechanisms, the « engines », converting these disequilibria into the entropy decrease characterizing life. The only truly empirical way to guess which kinds of engines may have operated at life’s emergence is to retrodict from what we see in extant living organisms. Let’s face it: studying bioenergetics means working with metalloenzymes! To the sole exception of quinones and flavins, the job of converting environmental redox gradients into pmf is performed by transition metals and clusters thereof, that is, by inorganic entities. Bioenergetics therefore fundamentally is an inorganic process. Intriguingly, several of the catalytic centres found in metalloenzymes strangely resemble metal-clusters observed in certain minerals (see figures below and Nitschke et al. 2013).
Let’s face it: studying bioenergetics means working with metalloenzymes! To the sole exception of quinones and flavins, the job of converting environmental redox gradients into pmf is performed by transition metals and clusters thereof, that is, by inorganic entities. Bioenergetics therefore fundamentally is an inorganic process. Intriguingly, several of the catalytic centres found in metalloenzymes strangely resemble metal-clusters observed in certain minerals (see figures below and Nitschke et al. 2013).
While the possibility of mineral-borne reactions having preceeded truly biological mechanisms was discussed in the past by Günter Wächtershäuser as well as by Michael J. Russell, examining the inventory of catalytic centres in metalloenzymes permits to narrow down the ensemble of promising candidate minerals and relevant reaction schemes having possibly played midwife in life’s emergence. Potential roles of specific minerals are discussed on our webpage on « Inorganic engines ».

References
Baymann, F., Schoepp-Cothenet, B., Duval, S., Guiral, M., Brugna, M.,
Baffert, C., Russell, M.J. and Nitschke, W. (2018)
Frontiers in Microbiology 9, 1357 [pdf-file]
On the natural history of flavin-based electron bifurcation
Wong, M.L., Charnaz, B.D., Gao, P., Yung, Y.L. and Russell, M.J. (2017)
Astrobiology 17, 975-983 [pdf-file]
Nitrogen oxides in early Earth’s atmosphere
as electron acceptors for life’s emergence
Bergdoll, L., ten Brink, F., Nitschke, W., Picot, D. and Baymann, F. (2016)
Biochim. Biophys. Acta Bioenergetics 1857, 1569-1579 [pdf-file]
From low- to high-potential bioenergetic chains:
Thermodynamic constraints of Q-cycle function
Duval, S., Santini, J.M., Lemaire, D., Chaspoul, F., Russell, M.J.,
Grimaldi, S., Nitschke, W., Schoepp-Cothenet, B. (2016)
Biochim. Biophys. Acta Bioenergetics 1857, 1353-1362 [pdf-file]
The H-bond network surrounding the pyranopterins modulates redox
cooperativity in the molybdenum-bisPGD cofactor in arsenite oxidase
Schoepp-Cothenet, B., van Lis, R., Atteia, A., Baymann, F., Capowiez, L.,
Ducluzeau, A.-L., Duval, S., ten Brink, F., Russell, M.J. and Nitschke, W. (2013)
Biochim.Biophys. Acta Bioenergetics 1827, 79-93 [pdf-file]
On the universal core of bioenergetics
Nitschke, W., McGlynn, S., Milner-White, J., and Russell, M.J. (2013)
Biochim.Biophys. Acta Bioenergetics 1827, 871-881 [pdf-file]
On the antiquity of metalloenzymes and their substrates in bioenergetics
Any attempt to rationalise how life may have originated on this planet necessarily requires an understanding of what life actually is. Over several decades, two fundamentally differing ways of defining life have emerged:
(1) The Inorganic versus Organic Chemistry Divide
Here life is defined by the chemical differences between « common sense » living things (i.e. mostly cellular entities) and bona fide inanimate matter eventually yielding the traditional list of crucial « organic » building blocks of life, that is, ribonucleotides, proteins and lipids. This line of reasoning ineluctably entails the necessity for these molecules to have been present at life’s origin resulting in Oparin and Haldane’s hypothesis of a « primordial organic soup » and consequently sparked the search for synthesis pathways of these molecules in an inorganic context. The presence of sufficiently high concentrations of the above mentioned 3 classes of organics (see Figure to the right), together with some ill-defined compartimentation-based concentration anisotropies, are then considered to be sufficient for life to originate. The further evolution into life’s present manifestations is often stipulated to pass through metabolisms based on catalytically active RNA-molecules (the « RNA-world hypothesis ») with a later take-over by polypeptide-based catalysts (proteins) and the role of ribonucleotides being reduced to inheritance. This is the narrative dominant in textbooks dealing with the origin of life.
However, this « From simple molecules to life »-narrative is a thermodynamic oxymoron. Chemists, physico-chemists and physicists (Schrödinger, Prigogine) have indeed long argued that an origin of life from such primordial organic soups is in blatant conflict with the 2nd law of thermodynamics which commands that any closed physical system evolves towards an increase in entropy, i.e. towards higher disorder. This is diametrically opposite to the notion of organic molecules spontaneously organising into higher order structures and eventually into living entities. Acute awareness of this problem led Jacques Monod to his assertion that life’s origin was so overwhelmingly unlikely that we necessarily are alone in the universe.
While the 2nd law thus essentially rules out prebiotic soup scenarios, it also shows a way out of Monod’s dilemma since a local decrease of entropy can indeed occur if (over-)compensated by fluxes of free energy. As noticed by Schrödinger, the essence of life and of its emergence therefore mustn’t be looked for in its building blocks but in the metabolic reactions converting environmental free energy into a decrease of entropy. Rather than relying on some ill-defined « special » properties of organics (a covert form of vitalism) in rationalising life, a truly scientific approach needs to consider life as a natural phenomenon and to search for the prerequisites making its emergence within the constraints of the physical world possible (Branscomb et al. 2017).
(2) The emergence of life from redox-energy converting reactions; Metabolism-first scenarios treat life as « another » self-organising far-from-thermodynamic-equilibrium phenomenon
Chemoautotrophic microorganisms take almost randomly distributed inorganic compounds (such as CO2, NH3, Fe, Mo, Ni, Cu etc.) and convert them into extraordinarily ordered molecules and supramolecular structures together with their corresponding reaction networks. They thus seemingly generate « organic » order from disordered inorganic elements and molecules without any need of input of organics from the environment. Such a decrease in entropy of course is only tolerated by the 2nd law if overcompensated by an influx of free energy from the « bath » the system is embedded in. Indeed, the above description of autotrophic organisms omits the crucial process allowing them to be « alive »:

ENERGY CONVERSION. Without the capacity to drive its internal entropy decrease by an entropy increase of the larger system, the existence and maintance of life would be unthinkable.
However, if persistent thermodynamic disequilibria are present in the environment, autocatalytic reaction schemes can be driven into self-organising, expanding chemical processes which represent the very essence of life while the precise elements/molecules involved are only « transducers » and not necessarily unique.
The universal thermodynamic equilibria tapped by life are redox gradients (Schoepp-Cothenet et al. 2013; see figure to the right). We therefore consider that some form of primitive redox-energy converting mechanism must have played midwife in life’s emergence.
References
Schrödinger, E. (1944) What is Life? The physical aspect of the living cell,
Cambridge University Press, Cambridge/UK (link)
Prigogine, I. (1980) From Being to Becoming: Time and complexity in the physical sciences,
WH Freeman (link)
Branscomb, E., Biancalani, T., Goldenfeld, N. and Russell, M.J. (2017)
Escapement mechanisms and the conversion of disequilibria; the engines of creation,
Physics Reports 677, 1-60 (pdf-file)
Schoepp-Cothenet, B., van Lis, R., Atteia, A., Baymann, F., Capowiez, L.,
Ducluzeau, A.-L., Duval, S., ten Brink, F., Russell, M.J. and Nitschke, W. (2013)
Biochim.Biophys. Acta Bioenergetics 1827, 79-93 [pdf-file]
On the universal core of bioenergetics
the Mineral/Metal-cofactor connectionAs we expand upon in our dedicated website on Energy and the Emergence of Life, the conversion of free energy is undisputably the most fundamental and indispensible process of life and has almost certainly been the crucial driving force for life’s emergence. An overwhelming majority of enzymes involved in the bioenergetic conversion of environmental redox disequilibria into « chemical energy » in the form of high intracellular ATP/ADP ratios are metalloenzymes, that is, their catalytic sites contain functionally essential metals or metal-clusters. This raises the possibility that life’s emergence was mediated by transition metals. Paraphrasing David Garner’s catchphrase « It is the inorganic elements that bring organic chemistry to life » we are tempted to say that « It is the transition metals that bring inorganic chemistry to life ».
Intriguingly, a number of metal-based cofactors catalysing redox reactions considered to be essential to early life strongly resemble specific minerals (see figure to the right, Nitschke et al. 2013, Russell et al. 2014). This observation provides strong support for Mike Russell’s « Alkaline Hydrothermal Vent Hypothesis » (first exposed in Russell et al. 1989) for life’s emergence. In this hypothesis, mineral-harboured transition metal centres would have performed the first metabolic redox reactions. To further assess the hypothesis of mineral-harboured redox catalysis pre-dating the processes occuring in metalloproteins, we have set out to experimentally test whether specific redox reactions occuring in extant bioenergetic mechanisms can be achieved using the corresponding, i.e. structurally resembling, minerals. We have chosen the mineral fougèrite as starting material and this project is directed by Simon Duval at BIP09 in collaboration with Fabienne Trolard at INRA/Avignon, Olivier Grauby at CINaM/Marseille, Bénédicte Menez at IPG/Paris and Mike Russell. References: Nitschke, W., McGlynn, S., Milner-White, J., and Russell, M.J. (2013) Russell, M.J., Barge, L.M., Bhartia, R., Bocanegra, D., Bracher, P.J., Branscomb, E., Kidd, R., McGlynn, S., Russell, M.J., Hall, A.J. and Turner, D. (1989) |
The Alkaline Hydrothermal Vent Theory for the Emergence of Life
The theory proposes that life was driven into being on our planet to resolve the disequilibria between the fuels H2 and CH4 emanating from submarine hydrothermal alkaline springs (pH ~11), as against the CO2 and NOx in the ancient atmosphere and dissolved in the acidulous ocean (pH ~5.5). The two fluids – one reduced and the other relatively oxidized – were kept at bay by the precipitation at the spring of iron minerals such as mackinawite (FeS) and the variable valence and physically pliable green rust ([FeII4FeIII2(OH)12][CO3]•3H2O) (left figure) (Branscomb and Russell 2018 part 2). It was in these mineral barriers that this free energy (redox and pH) was first converted, via a proto-metabolism, to organic molecules (Russell 2018) (right figure). Although today we recognize that life’s ‘job’ is to hydrogenate carbon dioxide, at its emergence it may have been primed through the oxidation of methane by nitrate to provide half of the reduced carbon (Nitschke and Russell 2013; Russell and Nitschke 2017). We may therefore expect life to emerge on any wet and rocky world that has a partly CO2-rich ocean (Russell et al. 2014). It should reveal itself either as whole cells or as bioorganic molecules that themselves are far-from-thermodynamic equilibrium. It is the waste from the low entropy feeds to life that keep it sustained and working. Indeed, life uses nano-engines such as Complex 1 and ATPase to couple exergonic reactions to endergonic ones (Branscomb and Russell 2018 part 1, Branscomb et al. 2017). Thus, life can be said to transcend chemistry, i.e., it can react hydrogen (or the electrons therefrom), generated geochemically as in hydrothermal convection, or photolytically as in oxygenic photosynthesis, to generate a small but ever continuous supply of very particular organic molecules on our, and most probably other comparable planets.
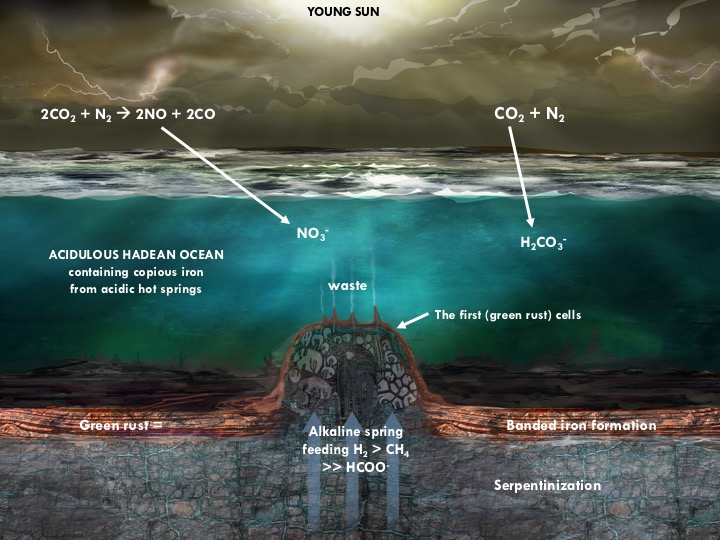
Figure 1: Diagram to show the redox and pH disequilibria driving the onset of metabolism. Volcanoes supply the CO2 and some NO. Lightning produces further NO (Ducluzeau et al., 2009; Nitschke and Russell 2009; Wong et al., 2017). ~410°C springs supply the metals to the ocean that precipitate as green rust (right figure) and minor mackinawite (Fe>>NiS) on meeting alkaline hydrothermal solutions at the hydrothermal mound and around its periphery (Russell, 2018; Branscomb and Russell, 2018 part 2). The green rust eventually converts into Banded Iron Formation. There are no continental landmasses in this Hadean water world as the radiogenically hot yet dense ocean crust comprising basalt, komatiite and iron formation, tends to founder and find a common level (Simplified from Branscomb and Russell, 2018 part 2).
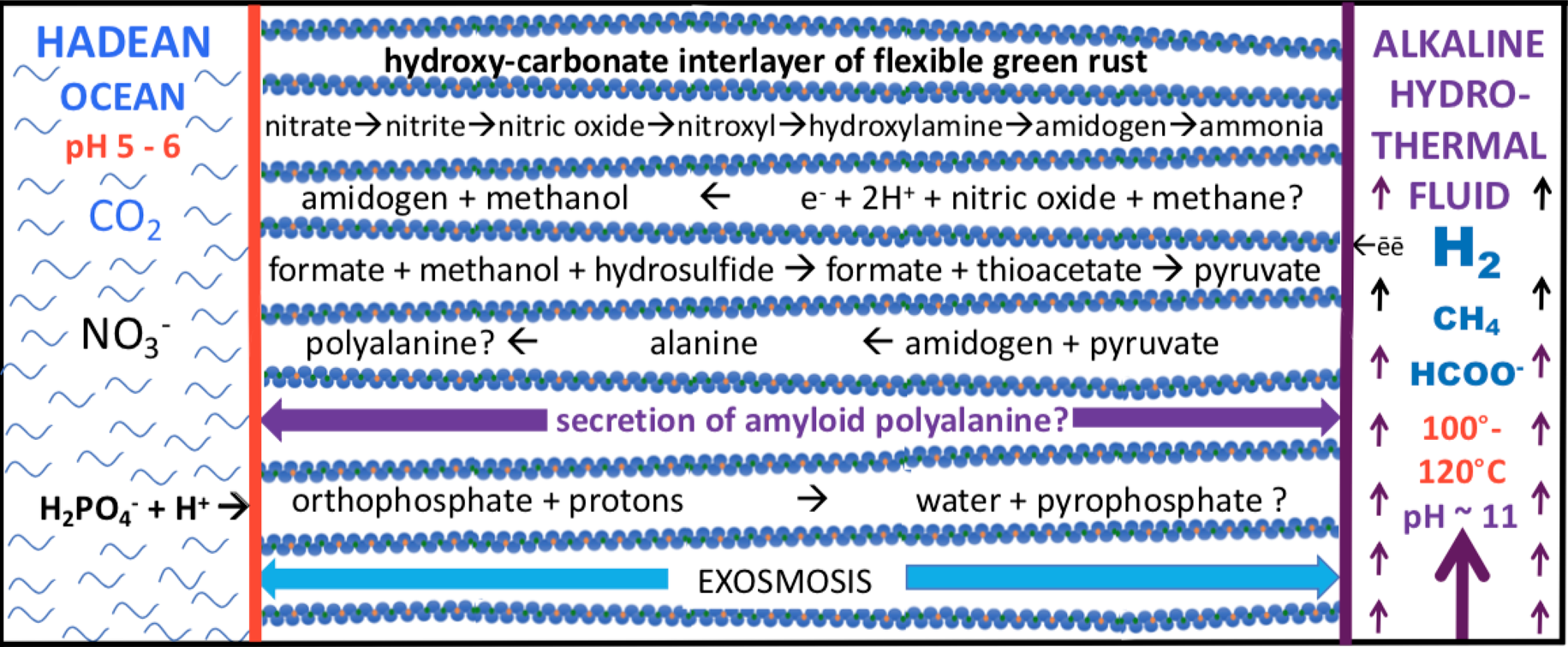
Figure 2: Sketch of some of the feed molecules and expected products driven by redox and pH (electron and proton) gradients as mediated within the nano-galleries of green rusts situated in, and comprising, a barrier between alkaline hydrothermal solutions and the carbonic Hadean Ocean (see right figure). Exosmosis, yet to be demonstrated, would obviate the need for wetting and drying cycles. Not to scale. Russell, 2018; Barge et al. 2019; Russell and Nitschke 2017; Nitschke and Russell, 2013)
Selected publications
Barge, L. M., Flores, E., Baum, M. M., VanderVelde, D. G. & Russell, M. J. (2019).
Redox and pH gradients drive amino acid synthesis in iron oxyhydroxide mineral systems.
Proc. Natl Acad. Sci. 116:4828-4833.
Branscomb, E. & Russell M.J. (2018)
Why the Submarine Alkaline Vent is the Most Reasonable Explanation for the Emergence of Life.
BioEssays, 41, 1800208.
Branscomb, E., Biancalani, T., Goldenfeld, N. & Russell M.J. (2017)
Escapement mechanisms and the conversion of disequilibria: The engines of creation.
Physics Reports 677:1-60. [pdf-file]
Branscomb, E. & Russell M.J. (2018)
Frankenstein or a submarine alkaline vent: Who is responsible for abiogenesis? Part 1: What is life – that it might create itself?
BioEssays 40(7) 1700179. [pdf-file]
Branscomb, E. & Russell M.J. (2018)
Frankenstein or a submarine alkaline vent: Who is responsible for abiogenesis? Part 2: As life is now, so it must have been in the beginning.
BioEssays 40(8). [pdf-file]
Ducluzeau, A-L, van Lis R., Duval S., Schoepp-Cothenet B., Russell, M.J. & Nitschke W. (2009)
Was nitric oxide the first strongly oxidizing terminal electron sink.
Trends in Biochemical Sciences 34, 9-15.
Nitschke, W. & Russell, M.J. (2009)
Hydrothermal focusing of chemical and chemiosmotic energy, supported by delivery of catalytic Fe, Ni, Mo/W, Co, S and Se, forced life to emerge.
Journal Molecular Evolution 69, 481-496.
Nitschke, W. & Russell, M.J. (2013)
Beating the acetyl coenzyme-A pathway to the origin of life.
Phil. Trans. R. Soc. Lond. B. Biol. Sci. 368, 20120. [pdf-file]
Russell, M.J. (2018)
Green rust: The simple organizing ‘seed’ of all life?
Life, 8, 35. [pdf-file]
Russell, M.J. & Nitschke, W. (2017)
Methane: Fuel or exhaust at the emergence of life.
Astrobiology 17:1053-1066.
Wong, M.L., Charnay, B.D., Gao, P., Yung, Y.L. & Russell, M.J. (2017)
Nitrogen oxides in early Earth’s atmosphere as electron acceptors for life’s emergence.
Astrobiology 17:975-983.
